4. Subjects, study designs, materials and methods
4.1. Human studies (Studies I - IV)
4.1.1. Subjects and study designs
All the subjects were adult volunteers, aged from 18 to 66 years. They were recruited mainly among the staff of the Institute of Biomedicine of the University of Helsinki, Finland, and their families and friends. In Study I subjects were also recruited by a local internet news group [rfc977] (puhe.terveys) which is mainly read by students and staff from Helsinki University of Technology, and through announcements among staff and students of the University of Helsinki. Additional subjects for Study IV were recruited by inviting all those who had participated in previous lactose intolerance studies (Vesa et al 1996, Teuri et al 1999) and had shown positive test results, indicating hypolactasia.
In Study I the inclusion criteria were either diagnosed lactose intolerance or a strong personal suspicion of it. Healthy subjects were invited as controls. All the volunteers, lactose intolerants as well as healthy controls, followed the whole test procedure of the oral lactose tolerance test. Of the volunteers in Studies II-IV, subjects were included if they showed positive test results, indicating hypolactasia, in two out of three lactose tolerance tests (the Golden Standard, see below). The tests used in Studies I - IV are shown in Table 4.1. Ten volunteers were excluded because they proved to be lactose digesters (II-IV). The final study group consisted of 72 subjects. Some of them volunteered for more than one study, so the total number of subjects in studies I-IV was 89 including those who withdrew or were excluded.
None of the subjects had received antibiotic (I-IV) or NSAIDs (IV) treatment during the 2 weeks preceding the studies, and they did not use any medication during the studies themselves. The subjects were interviewed by a physician, an authorized nutritionist, or a laboratory nurse, and they filled in a health questionnaire. Only those subjects who were seen to be healthy, with no recent history of gastrointestinal disturbances other than lactose intolerance, were included.
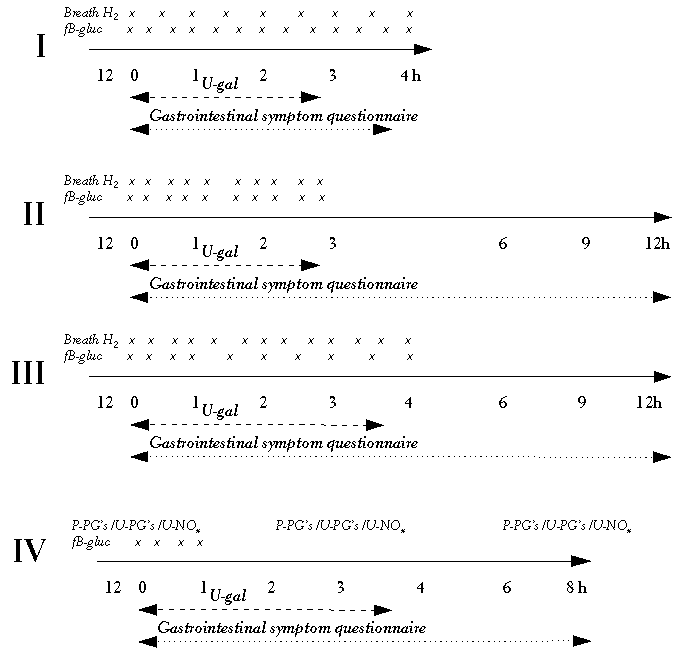
The first human study (I) was carried out as a method comparison study comparing two diagnostic instruments. The other human studies were carried out as randomised, double-blinded (II, IV), three - (II - III) or four-period (IV) cross-over trials with a 1-week wash-out period between the interventions. In each study in identical sessions the subjects were given lemon-flavoured test solutions, either 50 g lactose or 50 g digestible disaccharide sucrose in order to compare the effects of the two, in 300 ml water. The sampling sites for the biochemical measurements of the lactose breakdown products and other biochemical indicators and the recording of gastrointestinal symptoms were carried out according to the schedule of measurements (Figure 4.1).
Study I was designed to compare a small portable breath hydrogen analyser with the more commonly used equipment, and to compare both these with other possible test variables used in lactose tolerance tests.
In order to modify the gastric emptying rate (II), subjects ingested either propantheline (as bromide, 15 mg), metoclopramide (as hydrochloride, 10 mg) or an identical placebo (methylcellulose), all in capsule form an hour before the test solution. The doses of these drugs were chosen on the basis of previous studies and of the therapeutic single dose recommendation (Dollery 1991, Massicotte et al 1996).
In Study III, to investigate the role of the temperature of the test solution on the digestibility of lactose and the subjects' tolerance to it, the lactose was served either cold (2-3° C), at room temperature (20-21° C), or hot (55-58° C).
In order to test the role of prostaglandins (IV), the subjects ingested either 600 mg ibuprofen capsule to inhibit the synthesis of prostaglandins, or an identical placebo (methylcellulose) capsule with the test solution. In Study IV, to test the role of nitric oxide (NO), the subjects were on a low-nitrate diet for 48 h before the test days. All the capsules used in Studies II and IV were identical apart from the active ingredient, and were prepared at the University Pharmacy (University of Helsinki, Finland).
Table 4.1. Study designs and subjects.
|
Study I |
Study II |
Study III |
Study IV |
| L = Lactose, S = Sucrose, BMI = Body Mass Index |
| Interventions to be compared |
Breath hydrogen analysers for diagnosing hypolactasia |
L + propantheline
L + metoclopramide
L + placebo |
L cold
L room-temperature
L hot |
L + ibuprofen
L + placebo
S + ibuprofen
S + placebo |
| Study design |
Method comparison |
Three-period cross-over |
Three-period cross-over |
Four-period cross-over |
| Subjects (f/m) |
34/10 |
18/0 |
10/0 |
9/0 |
| Age, mean (range) years |
32 (18-66) |
34 (20-64) |
39 (26-55) |
38 (24-63) |
| BMI, mean (range) kg/m2 |
23 (19-33) |
23 (18-42) |
25 (20-42) |
22 (18-27) |
| Smokers |
5/44 |
3/18 |
3/10 |
1/9 |
| Lactose tolerance tests used: |
|
|
|
|
| Breath hydrogen |
+ |
+ |
+ |
- |
| Blood glucose |
+ |
+ |
+ |
+ |
| Urine galactose |
+ |
+ |
+ |
+ |
| Symptom questionnaire (h) |
4 |
12 |
12 |
8 |
4.1.2. Dietary instructions and study diets
The subjects were instructed to choose lactose-free food items for the 24 h preceding the test days (Studies I - III) and to avoid alcohol, smoking and foods that commonly produce gastrointestinal symptoms, such as beans, peas or fried foods, for the same period in order to diminish gastrointestinal symptoms induced by foods other than the test solution and to improve the reliability of the breath hydrogen measurements (Brummer et al 1985). In Study IV, in addition to instructions for lactose restriction, alcohol consumption and smoking, subjects were instructed to choose a low-nitrate diet for 48 h preceding the test days in order to clear the plasma of exogenously derived nitrate.
At fixed intervals from the beginning of the test procedure, the subjects in Studies II-IV were served a standard lactose-free lunch (after 3 h), an afternoon snack (after 6 h) and in Study II, dinner (after 9 h) (Table 4.2). In Studies III and IV the subjects were instructed to choose dinner from a selection of lactose-free items. This type of meal had been used previously in carbohydrate malabsorption studies (Rumensen et al 1990, Teuri et al 1999). Smoking was not allowed until the end of the test day, nor were coffee, tea or any other food items other than those provided.
Table 4.2. Content of meals served during the Studies II - IV.
| Food |
Study II |
Study III |
Study IV |
|
| Lunch 750 kcal (3.1 MJ) |
|
|
|
|---|
| minced meat |
+ |
+ |
+ |
| spaghetti |
|
|
+ |
| rice + carrots |
+ |
+ |
|
| wheat bread + margarine |
+ |
+ |
+ |
| blueberry puree |
|
|
+ |
| lettuce + canned peach |
+ |
+ |
|
| juice |
+ |
+ |
+ |
| banana |
|
|
+ |
| Afternoon snack 400 kcal (1.7 MJ) |
|
|
|
|---|
| wheat bread + margarine |
+ |
+ |
+ |
| cheese |
+ |
+ |
|
| banana + juice |
+ |
+ |
+ |
| decaffeinated coffee |
+ |
+ |
|
| Dinner 750 kcal (3.1 MJ) |
|
|
|
|---|
| minced meat + spaghetti + cheese |
+ |
|
|
| tomato |
+ |
|
|
| wheat bread + margarine |
+ |
|
|
| banana + juice |
+ |
|
|
4.1.3. Methods used for diagnosing hypolactasia and lactose intolerance
The Golden Standard diagnosis for hypolactasia and lactose intolerance (I-IV)
The lactose tolerance test was carried out by measuring the breakdown products of lactose following an oral dose - increase in exhaled breath hydrogen, unaltered concentration of blood glucose, increased excretion of urinary galactose - and the development of gastrointestinal symptoms. Between 7 and 9 a.m, after an overnight fast (10-12 h), the subjects were given 50 g lactose in 300 ml water, to be ingested in 5 min.
To avoid false positive or negative results in these studies, a Golden Standard diagnosis was established for this series of studies. The Golden Standard is a combination of the three diagnostic indicators: breath hydrogen, blood glucose and urine galactose. At least two positive indicators were considered sufficient to indicate hypolactasia. If lactose maldigestion was confirmed and gastrointestinal symptoms were considered to be at least moderate, the subject was diagnosed as being lactose intolerant.
Breath hydrogen measurement (I - III)
All the breath hydrogen measurements were carried out using a portable electrochemical hydrogen analyser, Micro H2 (Micro Medical Limited, Chatman, UK). Measurements were obtained by blowing (70 s) end-alveolar air, using 22 mm mouthpieces. Breath measurements were carried out as shown in Figure 4.1. An increase of ≥ 20 ppm was considered to be an indication of lactose maldigestion (see Arola 1994). In Study I, as a reference analyser for hydrogen production in exhaled air, the Quintron MicroLyzer, Model DP (Quintron Instrument Co. Inc., Milwaukee, WI, USA) was used.
Blood glucose and urine galactose measurements (I - IV)
All blood glucose concentration measurements were taken with the Glucometer Elite (Bayer Diagnostics, Lungby, Denmark). Before each new lot, of blood strips the equipment was auto-calibrated with a test strip of the new lot according to the instructions of the manufacturer. The accuracy of this blood glucose test method has been verified in earlier studies (Harrison et al 1996). Blood samples were aspirated directly from a finger tip according to the study designs, as shown in Figure 4.1. An increase in blood glucose concentration of 1.1 mmol/l or less was considered an indication of lactose maldigestion (see Arola 1994).
The subjects collected urine for the first three (I, II and IV) or four (III) hours, and urinary galactose was assayed spectrophotometrically using a commercial enzyme kit. If the 3- or 4-hour urinary galactose excretion was less than 20 mg, this was considered to be an indicator of lactose maldigestion (see Arola 1994). This method is considered to be a reliable, quantitative, non-invasive technique for assessing profiles of 'whole' intestinal lactase activity (Bjarnason et al 1990).
Symptom questionnaires (I-IV)
The severity of gastrointestinal symptoms was assessed by the subjects themselves on a scale of 0 to 10 (0 = no symptoms, 10 = severe symptoms) in a questionnaire which was slightly modified from previously used models (Vesa et al 1996, Teuri et al 1999). The subjects filled this in at the baseline before the intervention, and every 60 min for the first three or four hours after the intake of lactose, and then at 6 h, 9 h and 12 h (Figure 4.2). In Studies I and IV the recording of symptoms was completed at the end of four and eight hours respectively from the ingestion of the test solution.
The subjects assessed the intensity of flatulence, abdominal pain, abdominal bloating, borborygmi, nausea, headache, and the hardness of the stools. The sum of the most common lactose-induced symptoms (see Villako and Maaroos 1994) - flatulence, abdominal pain, abdominal bloating, borborygmi, and in Studies I and IV, loose stools - was calculated for each time point. For the sum of four symptoms the possible range was 0 to 40 and for the five symptoms the range was 0 to 50. If each symptom was calculated individually for the 12 h follow-up, the maximum score for each symptom would be 120. This long period of recording symptoms is important because of individual variability in the time of gastric emptying and intestinal transit and thus the development of gastrointestinal symptoms.
In Study II, in order to evaluate the usefulness of the numerical grading of symptoms, a visual analogue scale (VAS) (Figure 4.3) was used besides the numerical rating scale to see whether there were differences between these two methods. At the beginning of the test procedure subjects were given a numerical symptom questionnaire to be filled in during the next 12 h. After each of the first four test hours they were given another form, a new VAS questionnaire to be filled in, which they returned as soon as they had done so. After Study II the results of these two query methods were compared. The results did not differ. The 3-h symptom score (flatulence, abdominal pain, abdominal bloating, borborygmi) of the placebo period was 39.0 ± 4.7 in the numerical rating scale and 39.1 ± 4.6 in the VAS scale. The subsequent work of measuring the results with the VAS questionnaire is very laborious, and mainly for this reason the numerical rating scale questionnaire was chosen.
4.1.4. Gastrointestinal transit time (II)
Gastrointestinal transit time was measured using a carmine dye mixed with the lactose solution. The appearance of the dye was assessed visually in the stools by the subjects themselves (Read et al 1980, Marlett et al 1981).
4.1.5. Urine and plasma samples for inflammatory markers (IV)
The subjects collected urine for the 12 h before the test and over the next eight hours, divided into three aliquots for measurement of the excretions of prostaglandin E2 and prostacyclin metabolites (PGE2-M and 6-keto prostaglandin F1α respectively), and indicators of nitric oxide production (nitrite and nitrate and cyclic GMP). Venous blood samples for separating plasma were taken for prostaglandin E2 metabolite (PGE2-M) measurements, before the drug and carbohydrate intake (baseline) and 3 and 8 h after the baseline.
Plasma and urine levels of PGE2 metabolites were determined by using commercial radioimmunoassay kits following the instructions of the company. For measuring the indicators of nitric oxide production, nitrate was reduced to nitrite and determined spectrophotometrically by a commercial test kit based on the Griess reaction. For the cyclic GMP determinations, as another indicator of nitric oxide production, the urine samples were measured by radioimmunoassay as described by Axelsson et al (1988). The cyclic GMP antiserum has been described by Lähteenmäki et al (1998).
4.2. Animal experiments (V)
4.2.1. Experimental animals and study design
Female Wistar rats from the breeding colonies of the Laboratory Animal Centre, Helsinki University, Finland, were housed at 23° C with a light-dark cycle of 12 h.
Study V was designed to investigate the possibility that lactase protein expression and its activity can be induced by lactose. The expression of lactase protein in the gut and its possible induction was evaluated by immunohistochemical and biochemical techniques in 8-week-old rats (total number 24, divided into four groups) after a lactose challenge lasting seven days. The lactose-challenged rats received either 3%, 10% or 20% lactose-containing water or tap water (controls), ad libitum. The fluid intake was measured daily by weighing the drinking bottles.
4.2.2. Intestinal samples
The animals fasted overnight before they were killed by decapitation under CO2 anaesthesia. Two-cm-long samples for determination of lactase activity and expression were taken. Samples were from the proximal part of the stomach and the oesophagus, the middle of the duodenum, the proximal, middle and distal parts of the jejunum, and the proximal part of the ileum.
4.2.3. Biochemical determinations
Lactase activity
Lactase enzyme (EC 3.2.1.23) activity was assayed spectrophotometrically by the method of Dahlqvist (1964). Lactase activity was expressed as units (one µmol of lactose hydrolysed per minute at +37° C) per gram of total protein present in the homogenates. The protein content of the homogenates was assayed by the method of Lowry et al (1951).
Lactase expression by immunohistochemical methods
Paraformaldehyde (1%) fixed samples were immunoperoxidase labelled and incubated with monoclonal antibodies raised against rat lactase. Optical density measurements were used in order to obtain a semi-quantitative measurement of lactase protein expression.
4.3. Ethics
The designs of Studies I - IV were approved by the Ethical Committee of the Institute of Biomedicine, University of Helsinki, Finland. The design of Study V was approved by the Animal Experimentation Committee of the Institute of Biomedicine, University of Helsinki, Finland. Studies II and IV were also approved by the National Agency for Medicine, Finland.
4.4. Statistical analyses
In Study I, which was to compare the portable breath hydrogen analyser, the method of Bland and Altman (1986) was used to assess the agreement between the two breath hydrogen tests (Micro H2 vs Quintron).
In Study II, a mixed-effect ANOVA model was used to analyse the sum of symptom scores. Pair-wise post hoc analyses were carried out using repeated measures contrast analysis. The distributions of the symptom scores of individual symptoms in Studies II and III and the urine and the plasma variables in Study IV were skewed. Friedman's nonparametric analysis of variance was therefore applied to compare the differences between the interventions. The Wilcoxon matched pairs test was used for pairwise comparisons. In Study II, Spearman's rho correlation was calculated between the total transit time and gastrointestinal symptoms.
In Study V, statistical significance was determined by analysis of variance (ANOVA), and multiple comparisons were made by the Dunnet's post hoc test.
Sensitivity, specificity, and positive and negative predictive values were calculated to evaluate the breath hydrogen, blood glucose and urine galactose as diagnostic variables. The comparisons were made with the Golden Standard. Cohen's Kappa values and percentages of agreement were calculated to evaluate the agreement between any two diagnostic variables.
Results are expressed as Mean ± SEM, as Mean (range) or as percentage and 95 % CI. All statistical analyses were carried out using the SPSS statistical package (Release 7.5.1 / 8.0, SPSS Inc., Chicago, IL, USA).