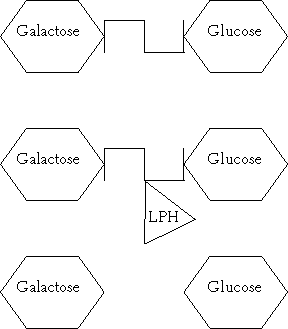
Figure 2.1. Lactose, which is made up of galactose and glucose molecules joined by β1,4 -glycosidic linkage, is hydrolysed by lactase (LPH).
The brush border of the intestinal epithelium (microvilli) contains glycoproteins which are responsible for the hydrolysis and absorption of dietary sugars. Lactose, the disaccharide of milk, consists of galactose joined to glucose by β1,4-glycosidic linkage (Figure 2.1). Before absorption this β1,4-glycosidic linkage must be hydrolysed by a microvilli enzyme called lactase (lactase-phlorizin hydrolase [LPH]).

Figure 2.1. Lactose, which is made up of galactose and glucose molecules joined by β1,4 -glycosidic linkage, is hydrolysed by lactase (LPH).
Lactase has two properties: lactase activity (β-galactosidase EC 3.2.1.23) and glucosidase activity (EC 3.2.1.62). The lactase site splits lactose and cellobiose, and some animal enzymes of this group also hydrolyse β-D-fucosides and β-D-glucosides. The glucosidase activity hydrolyses phlorizin, glycosylceramides and other aryl- or alkyl-β-glycosides, as reviewed by Keller et al (1993). However, lactose is the only substrate of significant nutritional importance.
Despite these two activities, lactase is a single polypeptide. It is synthesized as a large precursor of molecular weight, 205-245 kDa, and then processed to a mature enzyme of varying molecular weight, from 120-130 kDa in the rat to 160 kDa in the human, as shown in Figure 2.2 (Naim 1993). The variations in the molecular weights are probably due to the different glycosylation patterns in the various species (Naim 1993). The primary structure of a lactase molecule consists of 1927 amino acids in humans, 1926 amino acids in the rabbit and 1928 amino acids in the rat (see Keller et al 1993). Unlike sucrase-isomaltase, which is attached to the brush border membrane from the N-terminal end of the protein chain, lactase is anchored by a short hydrophobic C-terminal segment (see Keller et al 1993).
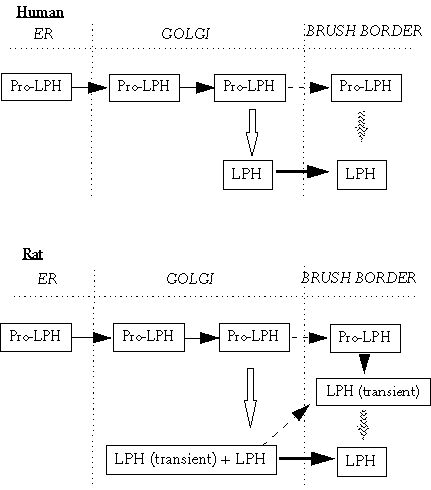
Figure 2.2. Synthesis of lactase in humans and rats. In humans, the lactase precursor (Pro-LPH) is synthetised and glycosylated in the endoplasmic reticulum (ER). In the Golgi it is converted to a complex glycosylated molecule and is cleaved by trypsin-like proteases (⇓). The final lactase (LPH) is transported to the brush border membrane. Some Pro-LPH molecules may not be cleaved and are transported to the brush border membrane and may be cleaved there by luminal proteases (![]() ). In the rat, Pro-LPH has two potential cleavage sites and may be cleaved to transient or final LPH in the Golgi. In the brush border membrane, uncleaved Pro-LPH and transient LPH are further cleaved to LPH (Naim 1993).
). In the rat, Pro-LPH has two potential cleavage sites and may be cleaved to transient or final LPH in the Golgi. In the brush border membrane, uncleaved Pro-LPH and transient LPH are further cleaved to LPH (Naim 1993).
Lactase exists only in mammals, but in other living organisms there are compounds which are related to it. Lactase-related β-glycosidases and phospho-β-glucosidases have been found even in eubacteria and fungi (see Keller et al 1993).
The relative activity of lactase is genetically determined and controlled by two alleles at a single gene locus (Swallow and Harvey 1993). The lactase gene has been located on chromosome 2q (Kruse et al 1988, Harvey et al 1993). In adults genetic polymorphism determines a high or low messenger RNA (mRNA) expression and activity (Harvey et al 1995). The mechanism for this polymorphism is not known but it is presumed that sequence differences in the gene determine whether or not lactose is down regulated (Wang et al 1998). Persistence of lactase (as measured by a lactose tolerance test) is dominant to non-persistence (hypolactasia) (Sahi et al 1973, Flatz 1987, Swallow and Harvey 1993). The activity in jejunal mucosa is less than 7 units/g protein in lactase nonpersistent subjects, and over 35 units/g protein in lactase persistent subjects, as reviewed by Arola (1994). The level of lactase activity in the heterozygotes is approximately half that of the persistent homozygotes (Swallow and Harvey 1993) but sufficient in most cases to hydrolyse fully a 50 g lactose load in a lactose tolerance test.
Most of the lactase activity in the rat develops late in gestation and stays at a high level from just before birth till the time of weaning. After that, within a few weeks the activity declines to the low levels of adulthood (Büller et al 1989). In the adult rat lactase mRNA and protein are abundant only in the middle segment of the intestine and are barely detectable in the duodenum and the ileum (Rings et al 1993). Studies on rats have suggested that intestinal lactase activity further declines in old age (Lee et al 1997).
The situation in humans is more complex. In the proximal small intestine of adults with high lactase activity, lactase protein and activity are present in all villus enterocytes. In hypolactasia lactase is patchily distributed on the villus enterocytes, and even enterocytes in the very same villus differ from each other (Maiuri et al 1992, Rossi et al 1997).
The genetically programmed down-regulation of the lactase gene is detectable in children from the second year of life (Wang et al 1998). In the Finnish population, the usual age of the onset of the clinically significant decline of activity varies within the 5-20-year range (Sahi et al 1972). Several factors have been implicated as the cause of lactase decline at weaning or in human hypolactasia. The reduction of (pre)pro-lactase synthesis has been associated with adult type hypolactasia (Witte et al 1990, Sterchi et al 1990, Lloyd et al 1990). A slow processing of the protein has also been reported (Witte et al 1990, Sterchi et al 1990). The major control mechanism is now thought to be at the level of mRNA (Rossi et al 1997, Wang et al 1998), but the heterogeneity of mRNA/activity ratio of lactase, even in a homogenous population, probably indicates that other mechanisms, besides transcriptional regulation, may be involved (Rossi et al 1997).
In lactase nonpersistence the activity of lactase in adults is about 5-10% of that found in early childhood (see Büller and Grand 1990). A very rare condition is congenital lactase deficiency (CLD) with an almost total lack of lactase (0-2% of activity of the enzyme at birth) (Savilahti et al 1983). CLD is part of the so-called Finnish disease heritage and the estimated incidence in the Finnish population is 1:60,000 births (Savilahti et al 1983). The gene locus for congenital lactase deficiency is found to be separate from but near to the lactase-phlorizin gene (Järvelä et al 1998).
The role of milk, and lactose as a component of milk, in modifying the expression of the lactase protein and its activity has been intensely studied during the past decades. At weaning, when diet is changed from a milk-based to a mixed adult diet, the small intestine undergoes functional maturation, as shown in many experimental studies with rats. Lactase activity decreases and its longitudinal distribution is modified, while the activity of other enzymes, such as sucrase-isomaltase (EC 3.2.1.10-48), increases. Intestinal maturation in rats depends on an intrinsic ontogenic programme (Duluc et al 1994) and on hormonal changes at weaning (Paul and Flatz 1983, Freund et al 1991). Nutritional changes have been shown to accelerate or delay the enzymatic decline and to modify the distribution of lactase mRNA in the small intestine (Lebenthal et al 1973, Duluc et al 1992, Nsi-Emvo et al 1994).
Experimental studies of the role of milk and lactose can be divided into two groups according to the age and the weaning stage of the animals. There have been numerous experiments to prevent the physiological decline of lactase expression and activity, by continued nursing, or by adding lactose to the diet immediately after weaning (Table 2.1). The diet of control groups varies from conventional rat pellets to a mixed diet with other di- or monosaccharides in the place of lactose. It is impossible to compare the actual doses of lactose because of the inadequate descriptions of the methods. The length of experimental periods varies from a few extra days nursed to several months with added lactose in the diet. The conclusion, however, is quite clear: dietary lactose, no matter what its source or the length of the test period, cannot prevent the physiological decline of lactase activity. In most of the studies, however, the reduction in activity is smaller in the lactose treated groups compared to the control groups on lactose-free diets.
| Species, age or weight | n | Dose and source of lactose | Length of experiment | Lactase A=activity, E=expression | Reference |
| + = Increase ; ± = no change / effect | |||||
| Rat, Sprague-Dawley, male, age ? | 23 | 25% lactose / corn starch in diet | 6-11 wk | A +25% | Fischer 1957 |
| Rat, Wistar, 30 - 60 d Germ-free / Conventional | 44 | Lactose, glucose or maltose (70.5 g / 100 g solids) | 4-8 wk | A +40% compared with glucose after 30 d | Reddy et al 1968 |
| Rat, Sprague-Dawley, 40-50 g | 16 | 5-60% lactose / lactose-free | 7 wk | A + | Cain et al 1969 |
| Rat, Wistar, 4 wk | <100 | 10% lactose / lactose-free | 24 ,32 and 40 wk | A + | Bolin et al 1971 |
| Rat, albino, 2 wk | 84 | Lactose / glucose 8% of total energy | 2-16 wk | A ± | Sriratanaban et al 1971 |
| Rat, age ? | 40 | Prolonged nursing / conventional lactose-free rat diet | 2-4 wk | A +120% | Lebenthal et al 1973 |
| Rat, Wistar, 1 d | 40 | 30% lactose / glucose + galactose | 2-11 wk | A ± | Leichter 1973 |
| Rat, Sprague-Dawley, 21 d | 30 | Powdered cow's / rat's milk 20% of diet (w/w) (1.6 - 2.5 g milk /day) / no milk | 0-10 wk | A ± | Becker et al 1974 |
| Pig, Chester White / Hampshire, 5 mo | 48 | 30% lactose (from dried whey) / corn starch in diet | 3 wk | A ± | Ekström et al 1976 |
| Rat, 0-27 d | 8 | Prolonged nursing | 4 wk | A & E +25% | Sakuma et al 1996 |
| Rat, Sprague-Dawley, 0-27 d | <50 | Prolonged nursing | 4 wk | A & E ± | Motohashi et al 1997 |
The number of experimental studies in which weaned adult animals were treated with a lactose-containing diet is smaller than that of experiments with preweaned animals (Table 2.2). The aim of these studies was to increase the low adult level of lactase activity in order to regain the higher level of sucklings. The conclusion of these studies was that dietary lactose can increase lactase activity to a level double the normal low adult level at the most, thus being about one fifth of the activity found in suckling animals.
| Species, age /weight | n | Dose of lactose (g / day) | Length of experiment | Lactase activity | Reference |
| + = Increase ; ± = no change / effect | |||||
| Rat, Wistar, female, 3 - 5 mo | 34/ 55 | Lactose / glucose 30% of diet | 2-31 wk | + >50% in jejunum with lactose and + 50% with glucose | Bolin et al 1969 |
| Monkey, adult | 7 | Lactose 20% (dry weight of diet) | 7 wk | + >30% in jejunum, + 70% in ileum |
Wen et al 1973 |
| Rat, 2 mo | 33 | Dose ? goat's milk |
1-3 d | + 100% in 24 h, no change after 72 h Added progesterone injections increased activity further |
Goldstein et al 1974 |
| Rat, Sprague-Dawley, female, 2 mo | 16 | 40% of energy as lactose / sucrose force fed | 1 wk | + 50% with lactose compared with low-carbohydrate diet +100% with sucrose compared with low-carbohydrate, high-fat diet |
Goda et al 1984 |
| Mouse, Swiss albino, age ?, 32-33 g | 48 | Milk, fermented milk or yoghurt 30% of diet (w/w) | 0.5-2 wk | + >25% in prox and >65% in distal jejunum after 3 d with fermented milk and yoghurt ± with milk diet |
Thoreux et al 1998 |
In human studies there have been only a small number of subjects in whom lactase activity was measured from small intestinal biopsies before and after the study period. None of the studies showed any increase in lactase activity after a daily oral load of lactose with increasing doses for ten days to 12 months (Cuatrecasas et al 1965, Newcomer and McGill 1967, Kreusch et al 1969, Gilat et al 1972). The number of subjects participating in these studies was three, two, 50 and ten respectively.
All these experimental (Tables 2.1 and 2.2) and human studies (Cuatrecasas et al 1965, Newcomer and McGill 1967, Kreusch et al 1969, Gilat et al 1972) to investigate those factors affecting or controlling the physiological decrease in lactase activity after childhood showed that dietary lactose did had minor effects. The same conclusion was drawn from a different angle in a study in which suckling mice were fed by transgenic α-lactalbumin-deficient females that produced lactose-free milk (Jost et al 1998). The feeding pattern was thus physiological. In spite of the lactose-free milk, the level of lactase activity and the longitudinal distribution of mRNA for lactase were unchanged compared to suckling animals nourished with normal lactose-containing milk, indicating that the effect of dietary lactose is small. There are no experiments to show how much lactase activity should increase in order to significantly reduce gastrointestinal symptoms in lactose intolerant subjects.
The possible effect of other dietary carbohydrates on lactase has mainly been studied with rat models. A high carbohydrate diet for one week, with corn starch forming 70% of the total energy and with no added lactose, increased disaccharidase activity (lactase, maltase and sucrase) in the rat jejunum (McCarthy et al 1980, Leichter et al 1984). Messenger RNA levels for lactase were elevated in rats fed a sucrose-enriched diet (Goda and Takase 1994) within 12 h of a carbohydrate intake (Goda et al 1999). This rapid accumulation of mRNA is thought to suggest that dietary sucrose enhances the efficiency of the transcription of the lactase gene.
Oat saponins (a mixture of avenacosides A and B) in vitro inhibited lactase activity (Önning and Asp 1995). This was not shown in in vivo studies in rats, probably due to far lower concentrations of saponins in their diets (Önning and Asp 1995). Saponins are thought to combine with the lactase enzyme and in this way to reduce the activity. Concentrations of saponins found in oat products probably have no effect on lactase activity in humans. Tannins have also been shown to reduce the lactase activity in rats fed on a diet containing 4 g tannins /kg body weight (Thomsen and Tasman-Jones 1982).
As shown by McCarthy et al (1980), a high fat diet is connected with low disaccharidase activity. Recently it was demonstrated that the saturation of dietary fats influences the activity of intestinal disaccharidases (Kaur et al 1996). Diets rich in saturated fats (coconut oil) increased lactase activity in adult rats compared to the control diet (commercial rat pellets). During a polyunsaturated fat diet (corn oil) or a fish oil diet lactase was not induced compared with the control diet. In piglets (Dudley et al 1994) the effect of the fat saturation level was the same as in rats.
Conflicting results of the effects of alcohol intake on lactase activity have been reported. In an experimental study with adult rats after three months of ethanol consumption (30% in drinking water [v/v]) lactase activity decreased (Rodriguez-Castilla et al 1996). The same effect has been seen in previous in vivo (Baraona et al 1974) and in vitro models (Dinda et al 1979), and also in one human study with alcoholic men (Perlow et al 1977). On the other hand, there are conflicting results in other experiments where no differences in lactase activity were found (Leichter 1987, Rodriguez-Castilla et al 1996). In fact, low concentrations (1-3%) increased lactase activity, at least in the epithelial cell line (Nano et al 1990).
Manipulating intestinal microflora by adding a probiotic (Probios(r), Pioneer Hi-Bred International Ltd, Johnston, Iowa, USA) to the diet of new-born piglets increased lactase activity in the piglets at three weeks old, but in the post-weaning period the differences in activity diminished and after about three months disappeared altogether (Collington et al 1990). Oral treatment of adult volunteers with lyophilized Saccharomyces boulardii for two weeks increased lactase activity, as measured by intestinal biopsy, by almost 80% compared to the basal activity (Buts et al 1986). The same effect was seen in adult rats on both viable and killed Saccharomyces boulardii doses for five days (Buts et al 1986). No morphological alteration of the intestinal mucosa was found either in humans or in rats. The possible reason for this yeast-induced increase could be the stimulation of protein synthesis at a translational level or the interference of proteolytic events of mature lactase by yeast cells, as Buts et al (1986) concluded.
Reports from the field of dietary components that may modify lactase expression and activity are fragmentary and unconsolidated. Only the surface of the subjects seems to have been scratched. Dietary patterns are changing, and the actual role of such factors as the whole diet or new dietary elements and functional foods on the capacity of lactase to digest lactose has nor been examined. There is a definite need for further investigation.
Lactose maldigestion may be caused by several diseases associated with injury in the small intestinal epithelium, such as chronic inflammatory bowel disease and Crohn's disease; infectious gastroenteritis, whether of viral (rotavirus), parasitic (giardiasis) or bacterial origin; and immunorelated injury such as sensitivity to gluten or immunodeficiency syndrome (HIV), as reviewed recently by Ushijima et al (1995) and Gudmand-Høyer and Skovbjerg (1996). A disease-induced decrease in lactase activity is usually temporary and can occur at any age. This is called secondary hypolactasia or secondary lactose maldigestion.
As seen above, lactase activity is depressed in celiac disease. The small intestinal mucosa from patients with celiac disease in remission synthesizes brush border membrane hydrolases like a normal ('healthy') mucosa. When challenged with gluten at a standard dose of 0.5 g/kg/day for one month, the tissue showed only slight mucosal damage, but the biosynthesis of brush border membrane hydrolases was reduced to the same level as in untreated celiac disease (see Lentze et al 1991). The lactase enzyme is said to be one of the slowest enzymes to recover (see Lentze et al 1991).
Lactase activity in adult rats has been increased by starvation for 48 h (Freund et al 1989) and 72 h (Leichter et al 1987). However, in obese lactose persistent human subjects, fasting resulted in the reduction of lactase activity (Knudsen et al 1968). Feeding lactose or glucose after a two-week fast in a two-subject trial did not increase lactase activity to the pre-fast level (Knudsen et al 1968). There seem, however, to be no differences in lactase activity/g mucosal protein between genetically obese mice (C57BL/6Jobob) and their lean controls, though there was difference in sucrase activity (Flores et al 1990). Total lactase activity was greater in obese mice because of their greater intestinal mass.
The field of drug interactions on lactase expression and activity has scarely been explored. Theoretically, any drug that impairs mucosal function or modifies its structure may have an effect on lactase expression and/or activity.
It has been common knowledge since the late 50s that the broad spectrum antibiotics such as neomycin, oral chlortetracycline and chloramphenicol reduce lactase activity (Faloon et al 1958, Sharma and Majudmar 1970). In a more recent study a low dose (i.p. 0.25 mg/g body weight) of actinomycin, which inhibits the transcription of genes, slightly increased lactase activity, but a high dose (i.p. 1.5 mg/g body weight) reduced this activity in hamsters in vitro (Andres et al 1985). A sucrose-induced increase in lactase mRNA can be reversed by the injection of actinomycin D (50 µg/kg body weight) in rats (Goda et al 1999).
Derivates of 1-deoxynoijirimycin and acarbose, which are α-glucosidase inhibitors, strongly inhibit sucrase activity without significantly affecting lactase (Lembcke et al 1985, Samulitis et al 1987). These types of α-glucosidase inhibitors have been developed for the treatment of metabolic and gastrointestinal disorders such as diabetes, obesity and the dumping syndrome (see Berger 1992).
In a series of short- and long-term experiments, Gill and co-authors showed the inhibitory effect of two widely-used histamine H2-receptor antagonists, ranitidine (0.1 - 1 mg/kg body weight) and cimetidine (0.003-2 mg/g body weight), on lactase activity in mice in vivo and in vitro (Gill et al 1989, Gill et al 1990, Gill et al 1991). They speculated that this adverse effect resulted from the chemical structure of these drugs and their interaction with the lipids of cell membranes, but this needs to be confirmed.
There are few studies of other drug-induced cases of the reduction or induction of lactase. Colcicine, which was previously used to treat acute gout, administered orally (50 µg/day) reversibly halved lactase activity in rats (Hudson and Smith 1986). In organ culture, the glucocorticoid agent dexamethasone stimulated the production of lactase in rats, but this effect was totally overturned by incubating the culture with cycloheximide (0.5 g/ml), a protein synthesis inhibitor (Hudson and Smith 1986). In hamsters, however, a dose of 1.5 mg/g body weight of cycloheximide produced no change in enzymatic activity in vitro (Andres et al 1985). The immunosuppressive agent cyclosporin A retarded normal maturation of the small intestine at the end of weaning, thus retaining lactase activity longer and delaying the physiological increase of sucrase and maltase activity in the rat (Cummins et al 1989).
As the studies referred to above show, further research is needed into drug-based modifications of small intestine digestion and capacity to absorb nutrients. Drugs affecting intestinal motility and thus the net absorption of lactose will be discussed later.
In lactose-digesting subjects, after the β1,4-glycosidic linkage between glucose and galactose has been hydrolysed by lactase, monosaccharides are actively transported through the epithelial cell. Galactose is absorbed more efficiently than glucose. Glucose enters the body glucose pool, but galactose is first metabolised to glucose, mainly in the liver (Leloir 1951) (Figure 2.3). The regulating enzyme of this pathway is UDP-galactose 4-epimerase. If galactose escapes hepatic metabolism, then it will either be metabolised by the enterocytes or be excreted in the urine (Henderson et al 1982). Galactose concentrations in urine are about 10 times higher than those in blood (Tengström 1968).
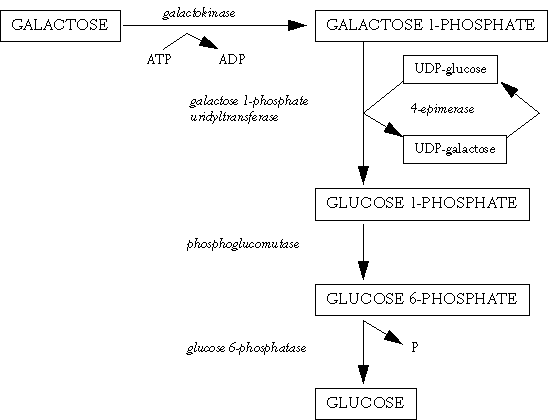
Figure 2.3. Galactose metabolism to glucose.
In lactose-maldigesting subjects immediately following a lactose challenge, an increased peristalsis was observed in jejunoscopy, at the same time as the mucosa became hyperemic and edematous (Banai et al 1984). Increased quantities of unhydrolysed lactose are present in the distal small intestine and the colon. The result of this is a significant osmotic pressure. Water and electrolyte secretion into the lumen increases. This osmotic flow will continue until equilibrium is reached (Launiala 1968).
The human colon epithelial cells do not absorb lactose as such. The colonic flora in each person is relatively stable but differs markedly between individuals (see Arola and Tamm 1994). The factors affecting the composition and activities of the colonic flora, and which could account for inter-individual variations, are largely unknown. One preliminary study showed that high concentrations of Escherichia coli tended to be associated with gas production in lactose maldigesters (Rautio et al 1999). Colonic bacteria, some of which have β-galactosidase activity, will metabolise a proportion of the lactose-producing short-chain fatty acids (SCFA) e.g. acetate, butyrate, propionate. Some of these acids, especially butyric acid, are absorbed by the colonic mucosa, to be used as substrate for the mucosa cells, but if the amount of SCFA exceeds absorption capacity, the residue is excreted in the stools, which will become acidic. The motor response of the colon to SCFA is complex. It seems that rather than inducing colonic transit time, at high concentrations SCFA may inhibit colonic motility and thus participate in the adaptation of the colon to its contents (Cherbut et al 1997).
The bacterial fermentation of lactose also produces gases such as carbon dioxide (CO2), hydrogen (H2) and methane (CH4). There are also large inter-individual variations in the activities of the flora that produces or consumes hydrogen. Excessive gas production causes abdominal distension, pain, borborygmi and flatulence. Excessive gas production and accumulation are strongly related to subjective symptoms (Hermans et al 1997). In a study with lactose maldigesters, the subjective symptoms did not correlate to the amount of malabsorbed lactose or to the volume or the rate of gas accumulation per se, but rather to altered intestinal transit and increased perception of luminal distension (Hammer et al 1996). These gases diffuse into the portal circulation and their concentrations in exhaled air will increase and can be used as an indicator of maldigested lactose.
The development of symptoms depends on the capacity of the colon to remove and use lactose and its fermented intermediary metabolic products. If this capacity is exceeded, gastrointestinal symptoms will develop. Women seem to be more liable than men to produce symptoms from similar amounts of malabsorbed lactose (Krause et al 1997).
The bacterial colonic adaptation of lactose maldigesters to a continued intake of milk or lactose (Johnson et al 1993b, Hertzler and Savaiano 1996, Briet et al 1997) or of totally unabsorbable carbohydrate lactulose (Flourie et al 1993, Florent et al 1985) has been reported. This may be due to changed acidity in the colon caused by unhydrolysed lactose. The metabolic production of gases is reduced with decreased acidity (Perman et al 1981, Holtug et al 1992), suggesting more effective fermentation. An increase in faecal β-galactosidase activity has also been found after continuous ingestion of lactose (Hertzler and Savaiano 1996). Rather than metabolic adaptation, Briet et al (1997) suggested that improved tolerance was just a placebo effect, because clinical improvement was also observed in the control group, which received sucrose.
The purpose of mixing and propulsive movements in the gastrointestinal system is to increase the contact of the luminal contents with the mucosal surface and to move the chyme along the tract. These movements are a result of the contraction and relaxation of the smooth muscle cells, which are arranged along the gastrointestinal tract longitudinally, obliquely (as in the stomach) and circularly (see Moffet et al 1993) (Figure 2.4).
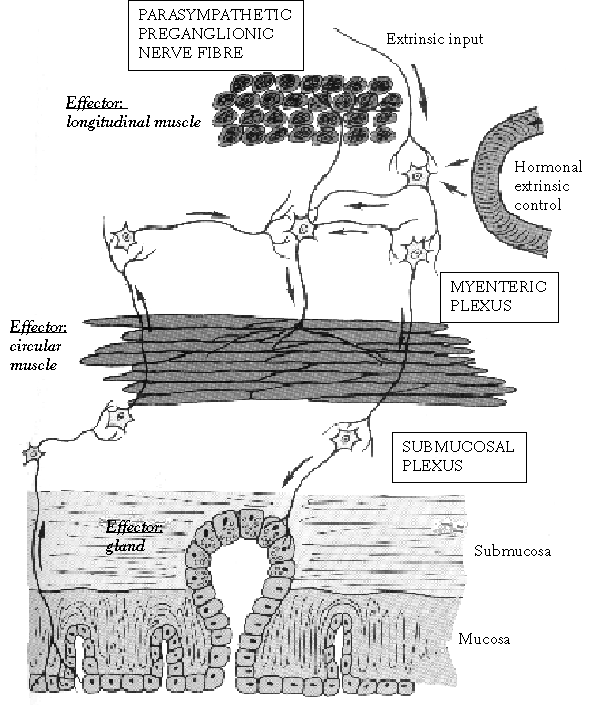
Figure 2.4. Enteric nervous system based on Moffet et al (1993). Even though the myenteric plexus primarily controls muscle contraction and the submucosal plexus controls secretion, they are extensively interconnected.
The main categories of movement are peristaltis and segmentation (see Moffet et al 1993). Peristaltis is composed of waves of contraction and relaxation of the longitudinal and circular muscle layers, resulting in the movement of chyme along the length of the tract. It is strongest in the swallowing pattern of the oesophagus, moderately strong in the stomach, and relatively weak in the intestine. Segmentation is the contraction activity of the circular muscle layer in order to mix the intestinal contents. The relationship and connection between propulsive and mixing movements is still under investigation (see e.g. Stevens et al 1999, Wood 1999).
Gastrointestinal motility is regulated by myogenic, neural and hormonal factors. In a fasting state the migrating myoelectric complex (MMC) passes along the intestine with the intense rhythmic contractions of the circular muscle (Szurszewski 1969, see Kunze and Furness 1999). It is followed by periods of less intense activity and rest. MMC clears the stomach and the small intestine of food remnants, intestinal secretions and other contents. It has been called the 'intestinal housekeeper' (Vantrappen et al 1977). The fasting motility pattern is interrupted by food and changed to continuous, irregular contractility.
The interdigestive MMC activity and the fed state activity are generated by the enteric nervous system and modified by extrinsic nerves. The enteric nervous system consists mainly of two plexuses (see Kunze and Furness 1999) (Figure 2.4). The myenteric plexus (Auerbach's) is located between the longitudinal and circular muscle layers, and the submucosal plexus (Meissner's) is in the submucosa. The enteric nervous system covers the whole gastrointestinal tract from the oesophagus to the anus. The myenteric plexus mainly controls gastrointestinal movements, and the submucosal plexus is responsible for gastrointestinal secretion and local blood flow. Even though the enteric nervous systems is an independent controller of the gastrointestinal system, the stimulus from parasympathetic and sympathetic systems can further activate or inhibit gastrointestinal functions. In addition, there are some reflexes from the gut that transmit signals for long distances in the gastrointestinal tract, such as gastrocolic reflex and enterogastric reflex (see Moffet et al 1993).
Understanding of different neurotransmitters released by the nerve endings is increasing (see Kunze and Furness 1999, Lindberg 1999). In addition to the classical neurotransmitters acetylcholine and noradrenaline a number of other transmitters are also known. In many studies to investigate possible regulators of MMC, such as insulin, opioids, calcitonin, motilin, and nitric oxide, some of these factors have been shown to act via the enteric nervous system and others via neural connections from the brain (see McConalogue and Furness 1994, Lindberg 1999). Nitric oxide may act as a messenger directly on the smooth muscle cells in regulating fasting intestinal motor activity (Russo et al 1999).
The motility effects of gastrointestinal hormones are minor to their secretory effects (Moffet et al 1993). Cholecystokinin is one with at least a moderate motility effect. It is secreted by the mucosa of the duodenum and the jejunum in response to dietary fats, fatty acids and monoglycerides. It releases bile into the small intestine by increasing the contractility of the gall bladder. It also moderately inhibits stomach motility and thus slows the emptying of the stomach, as does the gastric inhibitory peptide. Secretin is another gastrointestinal hormone with some slight effect on motility. It has mild inhibitory influences on most of the gastrointestinal tract motility. Motilin is released when the pH of the duodenal chyme is over 4.5. It facilitates digestion by increasing the strength of gastric contractions and the tone of the pyloric sphincter.
Visceral hypersensitivity has been recognized as being responsible for both motor alterations and abdominal pain in the pathophysiology of functional digestive disorders, particularly in the IBS (see Mayer and Raybould 1990, Mayer and Gebhart 1994, Bueno et al 1997). The role of afferent nerve pathways from the gut to the central nervous system have been emphasized, and it seems that in some IBS patients the pain threshold or response may be altered, and normally non-painful distension is sensed as being painful.
There are only a few studies on the sensitivity to pain of lactose intolerants. Whitehead et al (1990) compared tolerance to stepwise distension of a balloon in the rectosigmoid and to holding one hand in icy water, in irritable bowel patients, lactose maldigesters and asymptomatic controls. The lactose maldigesters had the lowest tolerance to icy water and the second lowest tolerance to balloon distension. On the other hand, in a recent study with previously carefully tested lactose maldigesters, an increased tolerance to ischemic pain was noticed compared to the asymptomatic healthy controls (Ylitapio 1997). In a questionnaire study these lactose maldigesters reported more frequent stomach pains than the controls, and the pain was more disturbing than to the controls. According to these studies it is possible that at least the lactose maldigesters possess reduced tolerance to visceral pain (visceral hypersensitivity), even though reports on tolerance to experimental pain produced by balloon distension or ice cold water have been conflicting.
Gastric emptying. The inhibition of gastric peristaltis, and thus the slowing of gastric emptying, by chemical or mechanical stimulation of the mucosa of the duodenum is called enterogastric inhibitory reflex. As there are receptors in the stomach and the duodenum which respond to volume, to osmotic pressure, to acids, fats, fatty acids and amino acids, and which thus control gastric emptying (see Cooke 1975, Malagelada 1990) many dietary manipulations have been carried out. By increasing viscosity or osmolality, and by increasing the energy, fat or carbohydrate content of test meals, gastric emptying has been delayed (Holt et al 1979, Foster et al 1980, Shafer et al 1985, Sandhu et al 1987, Vist and Maughan 1995).
It has been suggested that by delaying gastric emptying and thus increasing substrate mucosal contact time, the amount of undigested lactose can be reduced. This was investigated in studies where lactose was ingested in the form of milk with varying energy and fat contents (Welsh and Hall 1977, Dehkordi et al 1995, Vesa et al 1997a, Vesa et al 1997b), or as yoghurt (Marteau et al 1990, Arrigoni et al 1994, Mahe et al 1994), or with added ingredients such as chocolate (Welsh and Hall 1977, Dehkordi et al 1995), lactic acid bacteria (Dehkordi et al 1995), starch (Vesa et al 1997a), and fibre (Nguyen et al 1982), or as a part of a test meal (Solomons et al 1985, Martini and Savaiano 1988). All these factors are known to modify gastric emptying and thus the conclusion that lactose digestion is improved by retarding gastric emptying is justified. However, the relationship between pure lactose and gastric emptying, without the possible interference of the contents of milk or other dietary components, osmolality, viscosity or the consistence of diet has not been well documented.
The recent study of Barnet et al (1999), which is published only as an abstract, showed that previous exposure to lactose affected gastric emptying. The investigators speculate that this supports the inhibitory role of the intracolonic fermentation of lactose in the control of gastric emptying, which may explain frequent upper gastrointestinal symptoms in lactase nonpersistence.
Intestinal motility. Many factors that are known to delay gastric emptying also reduce intestinal motility and transit time. In many studies it is actually hard to distinguish the dietary effect on gastric emptying and on intestinal motility.
There are many studies investigating the role of dietary components in the interdigestive (postabsorptive) MMC. Food interrupts it and changes the motility pattern to a postprandial state. Even intravenously, certain nutrients, at least amino acids (Gielkens et al 1999), may modulate the interdigestive cycle of MMC. In one experimental study, the postprandial disruption of the MMC depended much more on the physiochemical composition of the diet than on its volume or energy content (De Wever et al 1978). In an other study, however, the caloric value of a meal regulated the duration of the fed state activity in the human small intestine without a 'physiological ceiling' of calories, at least for the normal caloric range per meal (220 - 1100 kcal) (Schönfeld et al 1997).
Of the dietary components, lipids are shown to have a stronger inhibitory effect on MMC than glucose, peptides or a mixed meal, when perfused with duodenal canula (Schang et al 1978) or eaten by conscious dogs (De Wever et al 1978, Eeckhout et al 1984). The infusions of nutrients (Schmid and Ehrlein 1993) or ethanol (Charles and Phillips 1995) in the proximal jejunum of dogs showed the same kind of change in the motility pattern as infusions in the upper parts of the intestine.
Coffee is said not to promote intestinal motility, as reviewed by Boekema et al (1999). Even if caffeine perfusion studies resulted in a net jejunal and ileal fluid secretion, no effect on small bowel transit time could be observed (Wald et al 1976). Nevertheless, Aranda-Michel and Giannella (1999) advise anyone suffering from diarrhoea to avoid caffeine-containing products because caffeine increases cyclic AMP levels and thus promotes the secretion of fluid, and may worsen the diarrhoea.
In the late postprandial state, the lower small intestine also regulates the proximal gastrointestinal motor function. In humans, intra-ileal perfusions with carbohydrates (Layer et al 1990, Gröger et al 1991, Layer et al 1993) or fats (Layer et al 1990, Layer et al 1993), simulating the late postprandial state, induce changes in the motility pattern of the intestine from the fed state to the interdigestive state, by activating MMC. These results are interesting because some of the carbohydrates, such as lactose, may be malabsorbed and thus reach the lower parts of the intestine under normal physiological conditions. However, in an experimental study with dogs, carbohydrate infusion of starch and glucose in a ratio of 3:1 in the proximal colon did not affect intestinal motility (Tohno et al 1995).
A very interesting study would be the intra-ileal perfusion of lactose and the subsequent measurement of its possible action on gastric emptying and upper gastrointestinal motility. In the study of Barnet et al (1999) this model is partly tested, but without the measurements of intestinal motility. There may also be a connection, other than the osmotic diffusion of water, between undigested lactose in the contents of the proximal intestine and the motility of upper parts of the intestine.
The consistency of the diet affects not only gastric emptying but also intestinal motility. In healthy humans the liquid part of a test meal (polyethylene glycol PEG 4000) appeared in ileal aspirates 1-2 h postprandially and always earlier than the solid part of the test meal (beans) (Kerlin and Phillips 1983). Ileal flow was shown to increase postprandially and to remain at a high level for at least 3 h.
In conclusion, dietary contents modify gastric emptying and alter intestinal motility on the whole length of the small intestine. The role of dietary delay on gastric emptying and lactose digestion has been well demonstrated. The effect of lactose on MMC and thus on intestinal motility has not been studied.
In many intestinal inflammatory diseases in which symptoms resemble those found in lactose intolerance, the endogenous synthesis of prostaglandins increases (see Hawkey and Rampton 1985, Rask-Madsen 1986). Prostaglandins are synthesised via the cyclo-oxygenase pathway (constitutive COX-1 and inducible COX-2) from arachidonic acid. They stimulate the contraction of the gastrointestinal smooth muscle and provoke many inflammatory responses such as vasodilation, vascular permeability and hyperalgesia (see Ooms and Degryse 1986, Rask-Madsen 1986, O'Loughlin et al 1991, Barrett and Bigby 1995).
If lactose-induced gastrointestinal symptoms are, at least partly, caused by local intestinal inflammation, the possible increase in the endogenous synthesis of prostaglandins after an oral load of lactose should be prevented by the inhibitors of prostaglandin synthesis and thus should reduce gastrointestinal symptoms. This has been investigated in only a few studies, with conflicting results. Premedication with 900 mg acetylsalicylic acid did not reduce lactose-induced symptoms in 12 lactose maldigesters (Flatz and Lie 1982). In previous studies, however, the inhibition of prostaglandin synthesis by acetylsalicylic acid (900 mg), indomethacin (25 mg), or ibuprofen (400 mg) reduced symptoms caused by incompatible food in three out of six patients (Buissert et al 1978). In two case reports, Lieb (1978, 1980) describes how a dose of 975 mg acetylsalicylic acid removed milk- and coffee-induced gastrointestinal symptoms.
Nitric oxide is another mediator which has been connected with inflammatory intestinal diseases. The substrate for the synthesis of this gaseous mediator is L-arginine, and its formation is catalysed by nitric oxide synthases (NOS). It modifies normal intestinal motility as described above as well as in an inflamed intestine such as in cases of Crohn's disease (Boughton-Smith et al 1993). The enhanced production of nitric oxide has been found in inflammatory-induced tissues (see Stark and Szurszewski 1992, Lefebvre 1995). The inducible form of NOS (iNOS) seems to protect the intestine from inflammatory injuries (McCafferty et al 1997). Prostaglandins and nitric oxide, both mediating the normal and inflamed motility of the intestine, seem even to co-operate, at least in regulating the immune response to injury (see Wallace 1996). The possible role of nitric oxide in lactose-induced symptoms has not been studied, as far as we know.
To conclude, prostaglandins and nitric oxide mediate inflammatory responses in the intestinal tract. The possible local intestinal inflammation caused by unhydrolysed lactose, and the role of prostaglandins and nitric oxide in the development of gastrointestinal symptoms, both need to be investigated in more detail.
The connection between the severity of gastrointestinal symptoms experienced in lactose intolerance and stressful life events has not been properly clarified. However, symptoms related to the passage of food through the gastrointestinal tract, such as abdominal pain and diarrhoea, are among the most commonly reported effects of acute life stress in patients with irritable bowel syndrome (IBS) (see Stam et al 1997). The symptoms of lactose intolerance resemble those of IBS, and on the basis of symptoms alone it is difficult to distinguish between these two states.
Patients with IBS often have exaggerated responses of gut motility in the small intestine (Camilleri et al 1989) and the colon (Fukudo et al 1993) in stressful situations. Colonic motility has been shown to increase after a stressful test, both in healthy controls and in IBS patients. Repeated tests, however, increased colonic motility only in the IBS patients (Narducci et al 1985). Cognitive therapy studies suggest that stress management procedures can alleviate the symptoms of IBS, including diarrhoea (Camilleri et al 1989).
The possible role of stressful life events and the expression of a changed intestinal motility pattern have been studied in healthy young men playing video games (Ditto et al 1998). The experimental stress reduced intestinal mean transit time, measured by a lactulose breath hydrogen test, by about 25 min compared with a relaxed situation. This was correlated with change in an index of cardiac sympathetic activity (Ditto et al 1998). However, psychological stress in medical students during final examinations did not change the orocecal transit time measured by an excretion of lactulose breath hydrogen test (Harris and Martin 1994). Gastrointestinal symptoms were also unchanged by the stress, except for abdominal pain, which increased. In healthy subjects gastric antral motor activity recorded with real-time ultrasonography was reduced by mental stress, but not in patients with functional dyspepsia who had reduced motility at baseline (Hveem et al 1996).
It is possible that the responses of gastrointestinal motility may vary depending on the stressor, as suggested by Rao et al (1998). They found that both psychological stress induced by a dichotomous listening test and physiological stress induced by keeping a hand in cold water enhanced colonic motor activity. Psychological stress affected stool propulsion and colon transit, whereas in healthy humans physical stress was associated more with delayed gut transit time (Rao et al 1998).
In experimental studies with dogs, acoustic stress for 1-2 h had no effect on fasting jejunal MMC, but prolonged the pattern of irregular contractions after a meal (Gue et al 1987, Gue et al 1989). When laboratory rats were partially or totally immobilised (restraint stress) their motility patterns were changed both in a fasted state and after a meal (Wittmann et al 1990). In the colon the changed motility pattern continued for three days. In rats colonic motility seems to be a more sensitive indicator of stress than the motility of the small intestine (Stam et al 1995). Autonomic pathways are thought to mediate stress effects on intestinal transit and motility (see Stam et al 1997), although responses are individual (Stam et al 1999).
To conclude, stressful stimuli modify visceral sensory and motor responses, a fact which is based mainly on experimental studies and the studies of irritable bowel disease. In IBS patients, stress increases the severity of gastrointestinal symptoms. Even though the pathophysiology of lactose intolerance differs from that of IBS, the symptoms do not, and thus it can be assumed that a stressful life also impairs, at least to some extent, the gastrointestinal symptoms of lactose intolerance.
Drugs which either deliberately or as an adverse-effect influence gastrointestinal motility may also have an influence on the digestion and absorption of lactose. Gastrointestinal prokinetics increase gut wall contractions, enhancing propulsive movements. They are used for the treatment of upper gastrointestinal disorders such as gastro-oesophageal reflux disease (see Horn 1996, Tonini 1996). Metoclopramide and cisapride are considered 'old' prokinetics. Metoclopramide has been used in the treatment of vomiting of different etiologies and for a wide range of functional and organic gastrointestinal disorders (see Koch-Weser 1981). Rather than having an antagonist effect on dopamine D2 receptors, metoclopramide seems to act via 5-HT4 (serotonin) receptors, to stimulate gastric emptying and enhance lower oesophageal sphincter pressure (see Tonini 1996). Cisapride is a serotonin (5-HT4) agonist, enhancing the release of achetylcholine and thus increasing lower oesophageal sphincter pressure and accelerating gastric emptying (see Horn 1996, Tonini 1996).
New prokinetic drugs are now being used in clinical studies. The 5-HT4 agonist prucalopride (R093877) has been found to accelerate colonic transit (Bouras et al 1999). Erytromycin is a macrolide antibiotic which disturbs gastrointestinal motility (see Alvarez-Elcoron and Enzler 1999). Its new structural analogues avoiding antibacterial properties are classified as motilin agonists and have been tested for inducing gastric contractions and reducing the motility of the small intestine (e.g. Sarna et al 1991, Caron et al 1996).
Some other antibiotics, such as the amoxicillin-clavulanate combination, may also in some cases disturb gastrointestinal motility, as shown by Caron et al (1991). Rather than disturbing the normal intestinal microflora, Caron et al speculated that this changed motility was due to the release of an intraluminal mediator such as motilin or to direct interaction with some aminobutyric receptors in the myenteric plexus. In addition to these, motility effects have been reported with somatostatin analogues (Dueno et al 1987, see Bueno et al 1997), the calcium channel blocker nifedipine (Konrad-Dalhoff et al 1991), gastric acid suppressants ranitidine, famotidine, nizatidine and omeprazole (see Bortolotti 1999), and the prostaglandin E2 analogue enprostil (Nicholl et al 1989).
As an adverse effect, many drugs may induce constipation. These drugs include agents from many categories such as analgesics, antacids (calcium and aluminium), anticholinergics, antidepressants, antihypertensives, diuretics, iron preparations, and neuroleptics (Tedesco et al 1985).
Laxatives enhance bowel movements by stimulating motility, retaining intraluminal fluid by osmotic mechanism, or by increasing luminal contents. Recent studies have shown that the pharmacological effects of some laxatives are mediated by prostaglandin release, increased mucosal permeability, epithelial injury, and the increased release of nitric oxide (see Izzo et al 1998). In addition to interest in the role of NO in the mechanisms of laxatives and in other effects of NO, a great deal of attention has been paid to the investigation of NO and the inhibitors of its synthese in regulating gut motility (see Tonini 1996). The inhibition of NOS may, however, result in undesired side-effects because of the widespread targets of NO.
Antidiarrhoeal agents reduce the number of bowel movements and diminish fluid loss. The most useful agents for slowing intestinal transit in acute diarrhoea are opiate derivates such as loperamide, diphenoxylate and bismuth subsalicylate (see Aranda-Michel and Giannella 1999). New peripherally-acting opiates such as fedotozine have been developed and tested as antidiarrhoeal agents and for the treatment of visceral hyperalgesia (Read et al 1997, Delvaux et al 1999).
Motility and sensory disturbances play an important role in the pathophysiology of the symptoms of IBS. In fact, the drugs used to alleviate acute symptoms of IBS are an overall collage of those agents affecting gut motility (see Bueno et al 1997, Paterson et al 1999). Constipation may be relieved with laxatives and diarrhoea with antidiarrhoeal agents. Attempts to resolve pain have been made by using drugs that reduce gut spasm, such as anticholinergics and smooth muscle relaxants, or prokinetics, to normalise the motility pattern of the intestine (see Paterson et al 1999), even if a clear relationship between symptoms and gastrointestinal motility has not been demonstrated (see Klein 1988). Potential drugs for modifying visceral hyperalgesia include opioid aginists (especially κ agonist fedotozine), serotonin antagonists (5-HT3 and 5-HT4 receptor antagonists), and possibly somatostatin and somatostatin analogues (see Farthing 1998). No medication has been shown to alleviate abdominal bloating or distension (see Paterson et al 1999).
There is only one published study, as far as we know, that describes the influence of a motility-affecting drug on the absorption of lactose. Lactose maldigesters were pre-treated with 8 and 12 mg loperamide on different days and lactose absorption was measured by using a breath hydrogen test (Szilagyi et al 1996). The loperamide-induced prolongation of intestinal transit time by about 30 min improved tolerance to lactose, as measured by reduced breath hydrogen concentration and diminished gastrointestinal symptoms. These results should, however, be confirmed in a double-blind placebo-controlled study.
To summarise, the primary cause for lactose intolerance, an unbalanced intake of lactose in relation to the activity of lactase, cannot be treated, at least so far, with motility-reducing or other drugs. Many drugs, however, have a prokinetic or antimotility action, and their use may either worsen or relieve the symptoms of lactose intolerance.
A variety of methods has been used for diagnosing lactose maldigestion. Precise standards do not exist. An intestinal biopsy is the only direct way of measuring lactase activity. This cannot, however, be regarded as a standard test for measuring tolerance to lactose, because mucosal lactase activity varies along the small intestine (Maiuri et al 1992, Rossi et al 1997) and thus lactase activity in one biopsy, from a restricted part of the small intestine, does not necessarily represent the total lactase activity of the whole small intestine. There seem not to be consistent cut-off values for hypolactasia. Varying activities between 5-18 U/g mucosal protein have been proposed (see Brummer et al 1993). The measurement of lactase activities from a biopsy is laborious, unpleasant for the patient, and can be performed only in hospitals.
In Finnish health care centres, measuring blood glucose concentration after an oral load of 50 g lactose is the most commonly used method for testing tolerance to lactose (Peuhkuri et al 2000a). At regular intervals, usually of 20 min, for up to 1-2 hours after the intake of a lactose solution, blood samples are taken and the glucose concentration is determined. A dose of 50 g lactose (in small children 2 g/kg body weight) is necessary for the blood test (see Arola 1994). Most laboratories regard a glucose increase of less than 1.1 mmol/l as an indication of lactose maldigestion. The test is simple to perform and the equipment needed is found in every clinical laboratory. However, the sensitivity and specificity of the test have been questioned because of the great inter-individual variations in gastric emptying and glucose metabolism (see Newcomer et al 1975).
Some laboratories use a lactose tolerance test with ethanol to improve the sensitivity of the test. Usually there are no measurable amounts of galactose in the blood or the urine, because absorbed galactose has been quickly metabolised to glucose in the liver (see Figure 2.3). In the tolerance test this change into glucose is inhibited with ethanol (50-150 mg/kg), thus enabling the measurement of the galactose concentration in the blood or the urine. 40 min after the ingestion of 50 g lactose, one capillary blood sample is taken. A galactose concentration below 0.3 mmol/l is considered an indication of lactose maldigestion (Isokoski et al 1972). Many scientists have questioned the need to use ethanol for measuring galactose in the blood and the urine in lactose tolerance tests, and modified methods without ethanol ingestion have been described (Arola 1988b, Grant et al 1989, Buttery and Ratnaike 1995). In a recent study, in 3-hour pooled urine samples, the sensitivity of a lactose tolerance test without ethanol was 100% and specificity was 94%, (Alvarez-Coca et al 1996).
The measurement of a breath hydrogen response after an oral load of lactose is based on the principle that unhydrolysed lactose is fermented by the colonic microflora, producing hydrogen and methane. Gases are diffused into the blood circulation and excreted in expired air (Levitt 1969). A dose of 25 g (in children 1 g/kg body weight) has recently been demonstrated as being sufficient in a breath hydrogen test (see Hamilton 1998). This dose produces almost the same response in breath hydrogen concentration as the larger dose of 50 g. The hydrogen concentration is analysed either by gas chromatography or by an electrophysical sensor (Bartlett et al 1980). Breath samples are taken at regular intervals of 30 min after the ingestion of lactose until the hydrogen concentration exceeds the baseline value by at least 20 ppm, indicating lactose maldigestion, or for up to three hours (see Hamilton 1998). A recent study by Karcher et al (1999) suggested that a better cut-off value would be 10 ppm because the patients with only moderately increased hydrogen excretion (10-20 ppm) may not be lactose deficient at all, as Karcher et al speculated.
The simultaneous measurements of breath methane may increase the reliability of a tolerance test because some types of methanogenic bacteria in the colon convert colonic hydrogen to methane. Bjørneklett and Jenssen (1982) found that 44% of 120 healthy subjects were methane producers. Thus, there seem to be hydrogen producers, methane producers and those who produce both hydrogen and methane. There do not seem to be even rough estimations of the number of pure methane producers, i.e. the subjects who produce no hydrogen at all. If breath methane alone is analysed, an increase of about 12 ppm is considered an indication of lactose malabsorption without reference to the hydrogen response (see Hamilton 1998). The breath methane test alone has lower sensitivity and specificity than the breath hydrogen test and cannot replace the latter (Myo-Khin et al 1999).
The lactose breath hydrogen test is considered to be fairly reliable in detecting lactose maldigestion (see Arola 1994, Hermans et al 1997, Hamilton 1998). There are some factors that may possibly increase breath hydrogen excretion and may thus reduce the reliability of the test, as reviewed by Arola (1994) and Hamilton (1998). The influence of these factors is reduced when labelled lactose is used. After the ingestion of 13C-labelled lactose (non-radioactive isotope), the digestion and absorption of lactose can be detected as the cumulative concentration of labelled CO2 in the breath (Newcomer et al 1975, Hiele et al 1988, Vantrappen et al 1992). Nowadays there is no risk of radiation, as there was in an earlier modification of the use of a 14CO2-labelled malabsorption test (Salmon et al 1969, Sasaki et al 1970). In a recent study by Koetse et al (1999), the sensitivity and specificity of a combined 13CO2/H2 breath test were 85% and 65% respectively.
The relationship varies between these tolerance tests and gastrointestinal symptoms. There is a stronger association between the amount of hydrogen excreted and gastrointestinal symptoms, whereas that between the increase in glucose concentration and the symptoms of lactose is less evident (Newcomer et al 1975, Hermans et al 1997, Teuri et al 1999). The blood glucose test is the most commonly used in Finland (Peuhkuri et al 2000a).
It seems probable that the development of gastrointestinal symptoms caused by maldigested lactose depends on the function of the whole gastrointestinal tract, not just on the digestive and absorptive capacity of the upper small intestine.
Firstly, according to several studies, small doses of lactose, of up to 10-12 g, are tolerated by many lactose maldigesters without gastrointestinal symptoms (Suarez et al 1995, Vesa et al 1996). If a daily dose of lactose is divided into several small portions, to be ingested throughout the day, the possibility of symptoms will decrease. This will lead to an improved balance between the hydrolysing capacity of the enzyme and the load of ingested lactose.
Secondly, other components of food ingested with lactose, as discussed above, delay gastric emptying and intestinal motility, and this has been shown to improve tolerance to lactose. If lactose is ingested in a reasonable quantity as a part of meal, the possibility of symptoms, and/ or their severity, may decrease.
Thirdly, it may be possible in the future to reduce lactose-induced gastrointestinal symptoms simply by supporting the function of the colon and its bacteria.
Finally, the possibility of secondary lactose intolerance, induced either by gastrointestinal or other diseases or by their treatments, drugs, a stressful lifestyle or any other factor, should not be overlooked.